Introduction
The Autonomic Nervous System (ANS) is a complex network of nerves and ganglia which work to regulate a variety of sensory and motor systems without conscious effort (Berntson 2006). It is separated into two branches, the sympathetic and parasympathetic nervous systems. These two branches have very clear points of origin and differ in their functions. As you can see in Figure 1, the sympathetic system is centralized in the intermediolateral cell columns in the thoracic and lumbar divisions of the spinal cord. The parasympathetic system’s center of origin resides in the brainstem and the lower sacral division in the spinal cord.
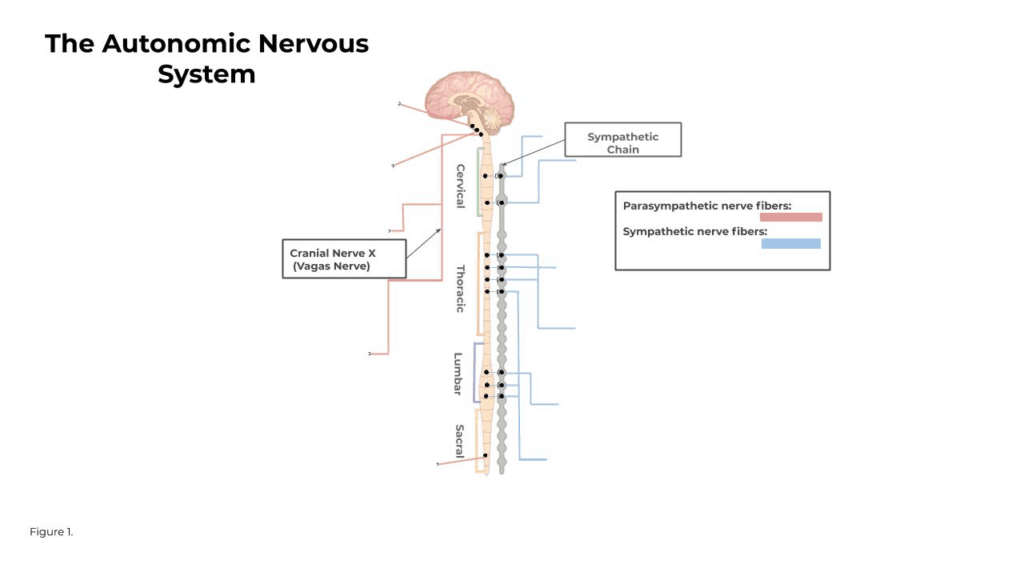
In the past, it was thought that the activity of the sympathetic and parasympathetic branches were reciprocal, reflexive, and non-specific across target organs. It is now clear that this was an inaccurate and oversimplified depiction of the ANS. Although many regulatory functions of the ANS are basic reflexive responses managed by lower neural substrates, higher neural substrates like the cerebral cortex (where most information processing in the brain occurs) have also been closely linked to having an effect on ANS activity (Berntson 2006) . So the neural substrates that regulate ANS functions are the same ones controlling cognitive, behavioral, and affective processes. We now know that the parasympathetic and sympathetic branches can operate independently from one another, especially in psychological contexts, and responses can be isolated to a single target organ. (Berntson 1991)
Parasympathetic and Sympathetic Nervous System
As shown in Figure 2, the parasympathetic branch is generally known as the brakes and slows many functions of the body.
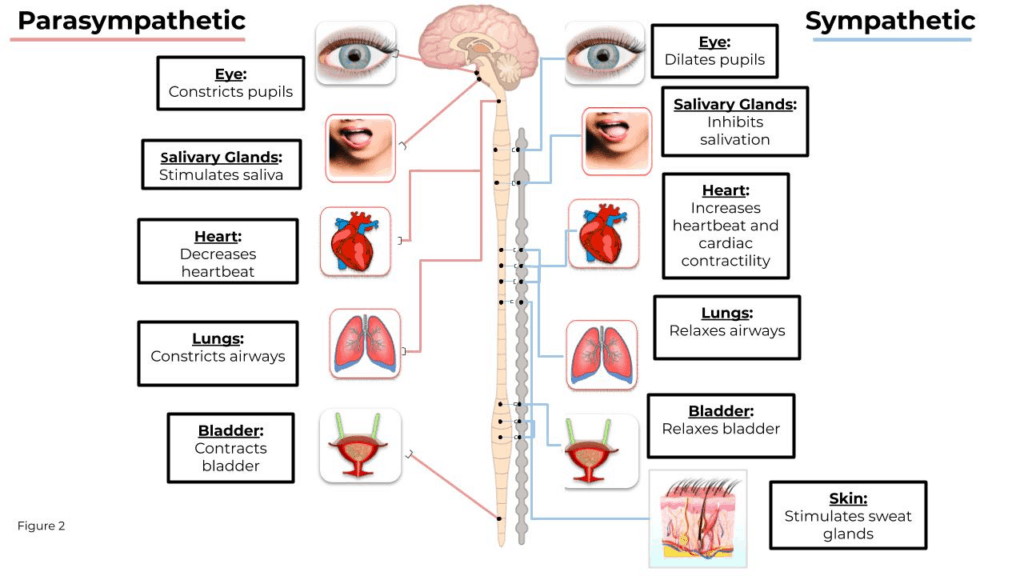
But it does not act like the brakes in every occurrence of activation – in the case of the salivary glands and the stomach, the parasympathetic system stimulates both those organs. The sympathetic system, conversely, accelerates the body systems and gives them the needed energy for a fight or flight response (De Maria 2000). The complexities and interactions of the systems make it necessary to focus on both systems when gaining an understanding of the responses of the body in reaction to different stimuli. In the following section, we will cover the three most widely used and validated measures of the ANS.
How to Measure the ANS
Heart Rate Variability
Heart Rate Variability (HRV) is a measure of the variation in the time interval between subsequent heartbeats, or interbeat intervals.
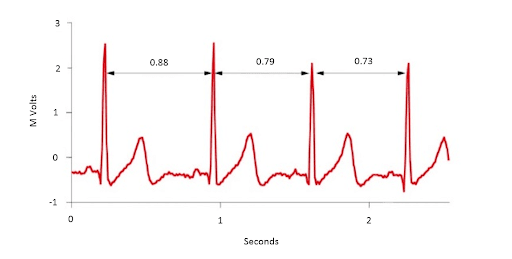
HRV is impacted by both the parasympathetic nervous system and the sympathetic nervous system and is generally related to emotional arousal. The parasympathetic nervous system works quickly to decrease heart rate, while the sympathetic nervous system works slowly to increase heart rate, and this is important because it applies to different psychological states. By isolating a specific frequency range of variability, referred to as High Frequency HRV (HF HFV), we can derive a pure index of parasympathetic nervous system activity.
To measure this phenomenon, an Electrocardiogram (ECG) is used to measure the fluctuations in the electrical activity of the heart. ECG is considered to be the best tool for measuring HRV because it produces a waveform that is very clear which makes it easier to ensure accurate beat timing and exclude heartbeats not originating in the sinoatrial node. Following the detection of beats and a series of transformations, variability in the High-Frequency (HF) range (typically 0.12 (or 0.15)-0.40 Hz) can be isolated to obtain a measure of parasympathetic activity. Activity in this range is associated with the respiratory sinus arrhythmia (RSA), a vagal mediation of heart rate such that it increases during inspiration and decreases during expiration. (Hill 2009) High-frequency (HF) activity has been found to decrease under conditions of acute time pressure and emotional distress, and an elevated anxiety state. Click here to learn more about HRV, it’s origins, and how variables are calculated.
Impedance Cardiography
Increasing interest among psychophysiologists in sympathetic influences on the heart has created the need for noninvasive techniques for analyzing these influences. (Newlin, David B. 1979). Impedance Cardiography (ICG) converts changes in thoracic impedance (as measured by electrodes on the chest and neck) to changes in volume over time and allows tracking of volumetric changes such as those occurring during the cardiac cycle. This provides visibility into blood flow in the heart non-invasively. Using the ICG signal along with ECG, a number of interesting systolic time intervals can be observed. One of which, Pre-Ejection Period (PEP), is a pure measure of sympathetic cardiac control. PEP is the time elapsed between the electrical depolarization of the left ventricle (QRS on the ECG) and the beginning of ventricular ejection (B on dZ/dt) and represents the period of left ventricular contraction.
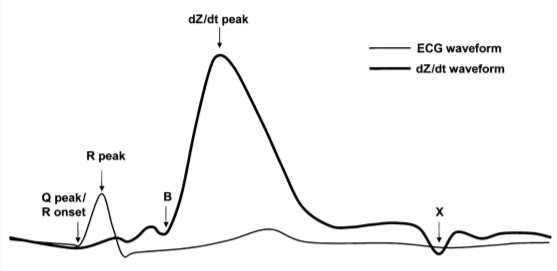
By combining the ECG signal with ICG, the effects of the parasympathetic and the sympathetic nervous systems can be derived on the same target organ. How the target organ (heart) is impacted by both of these systems can give you a more complete idea of how the regulatory systems respond to emotional arousal. Click here to learn more about ICG.
Electrodermal Activity
Electrodermal Activity (EDA) is the measurement of skin resistance which varies with the state of eccrine sweat glands in the skin. These sweat glands are innervated by the sympathetic nervous system, therefore skin conductance can be an indication of psychological or physiological arousal. If the sympathetic branch of the ANS is highly aroused, then sweat gland activity also increases, which in turn causes the skin to become more conductive.
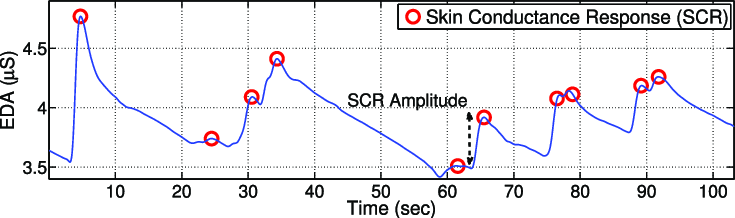
Skin conductance is not under conscious control. Instead, it is modulated automatically by sympathetic activity which drives human behavior, cognitive and emotional states on a subconscious level. Skin conductance, therefore, offers direct insights into autonomous emotional regulation. To learn more about EDA you can click here.
Summary
The ANS is a complex system dedicated to maintaining a homeostatic state. Because of the complexities and intricacies of both branches of the ANS, measuring sympathetic and parasympathetic activity in the same organ allows for more direct comparison and model generation which is essential if the goal is to use these measures to better understand the psychophysiology of cognitive, affective, and behavioral processes. When possible, therefore, it is recommended to measure cardiac autonomic regulation through heart rate variability and impedance cardiography.
References
1Berntson, Gary G., John T. Cacioppo, and Karen S. Quigley. “Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint.” Psychological review 98.4 (1991): 459.
2Berntson, Gary G., et al. “Autonomic space and psychophysiological response.” Psychophysiology 31.1 (1994): 44-61.
3Berntson, Gary G., Martin Sarter, and John T. Cacioppo. “Autonomic nervous system.” Encyclopedia of cognitive science (2006).
4Cybulski, Gerard, et al. “Impedance cardiography: recent advancements.” Cardiology journal 19.5 (2012): 550-556.
5Cacioppo, John T., and Gary G. Berntson. “Integrative neuroscience for the behavioral sciences: Implications for inductive inference.” Handbook of Neuroscience for the Behavioral Sciences (2009).
6 De Maria, Anthony N., and Ajit Raisinghani. “Comparative overview of cardiac output measurement methods: has impedance cardiography come of age?.” Congestive Heart Failure 6.2 (2000): 60-73
7 Hill, L. K., et al. “Are all measures created equal? Heart rate variability and respiration.” Biomed. Sci. Instrum 45 (2009): 71-76.
8 Lozano, David L., et al. “Where to B in dZ/dt.” Psychophysiology 44.1 (2007): 113-119.
9 Newlin, David B., and Robert W. Levenson. “Pre‐ejection period: Measuring beta‐adrenergic influences upon the heart.” Psychophysiology 16.6 (1979): 546-552.
